Sequence WebPatient is a Full Featured, Cloud-Based Solution that is Investigator Site Friendly, Fully Configurable and Promotes Fast Study Start-Up by Streamlining Patient Eligibility Assessment and Recruiting.
- AI Powered with Advanced Learning Algorithms
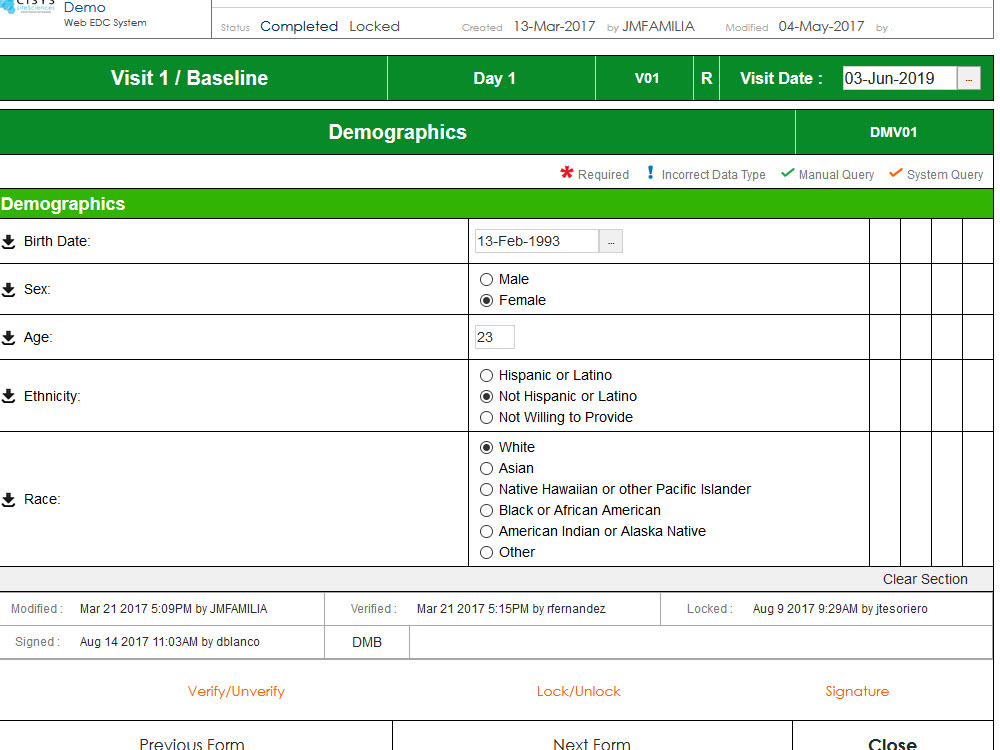
- Easy to use, feature rich forms and functions for evaluating Patient Eligibility for a study
- On-The-Spot, algorithm based indication of eligibility
- CRO/Sponsor eligibility review workflows
- Fast, automated communication of patient data and eligibility status across the research team and all desired personnel
- Extensive query management system to eliminate email, manage communications, protect patient data, maintain audit trails and follow 21 CFR Part 11 guidelines
- Email/Text/Query notifications can be configured to trigger on any data point or combination of data points
- Flexible PDF archiving of eCRFs/audit trail for study closeout
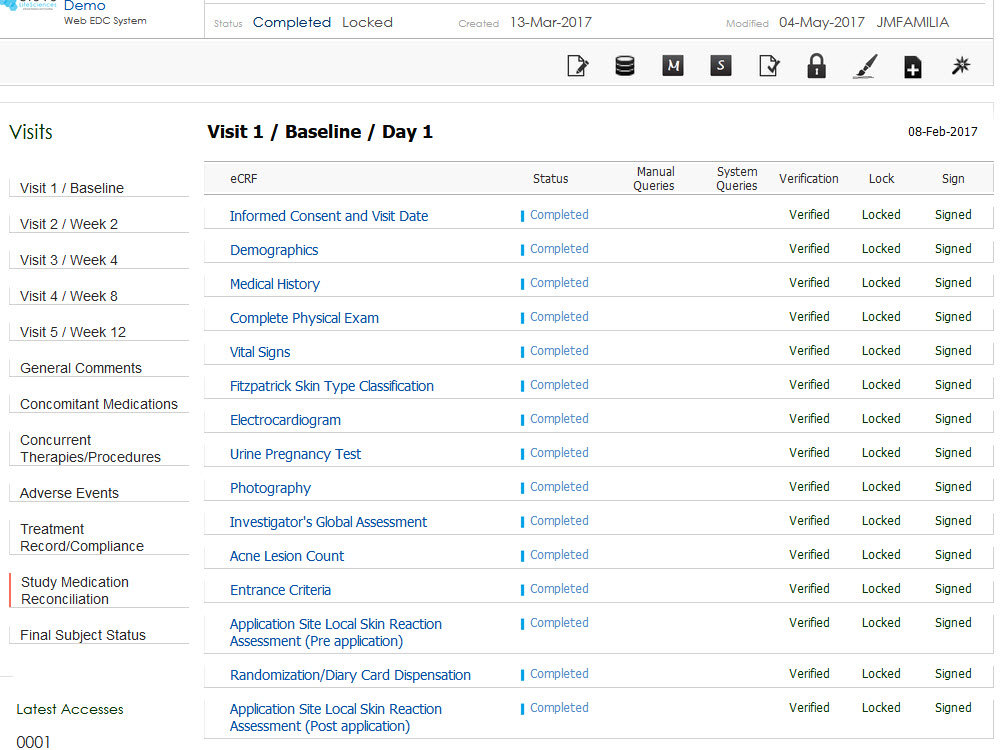
- Interactive task-based dashboards and real-time reporting
- Greatly improves recruiting efficiency
- Promotes faster study execution
- Documents eligibility decisions and communications
- Requires minimal training with user friendly GUI
- Highly configurable, standards based
- Can be integrated with most EDCs or used as a stand-alone coding solution
- CDISC / CDASH standards
- 21 CRF Part 11 Compliant
Support and Training
- Quick and efficient user training
- In-person training also available onsite at sponsor facilities
- Zero client requirements except browser
- Remote training available
- Technical support and dedicated 800 number per study
- Many levels of help desk and support options available
Security
- Role and workflow-based security
- Customizable event notifications
- Configurable workflow to fit customer’s organization
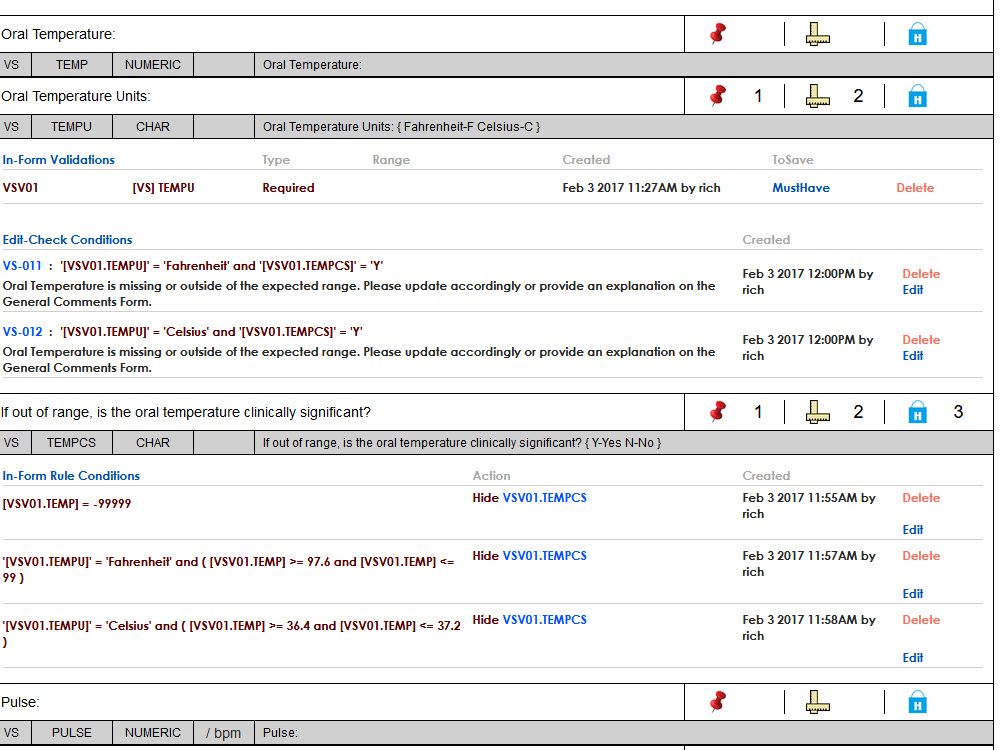
- Configurable down to the field level
- Configurable actions by role or user
Data / Hosting
- All data will be hosted on a validated, secure server located in a hardened, redundant hosting facility
- Full business continuity and disaster recovery functions provided
- All data will be backed up on a nightly basis both locally and to a different geographic location for redundancy
- 24/7/365 Performance and fault monitoring
- All files will be the client’s property and transferred upon request