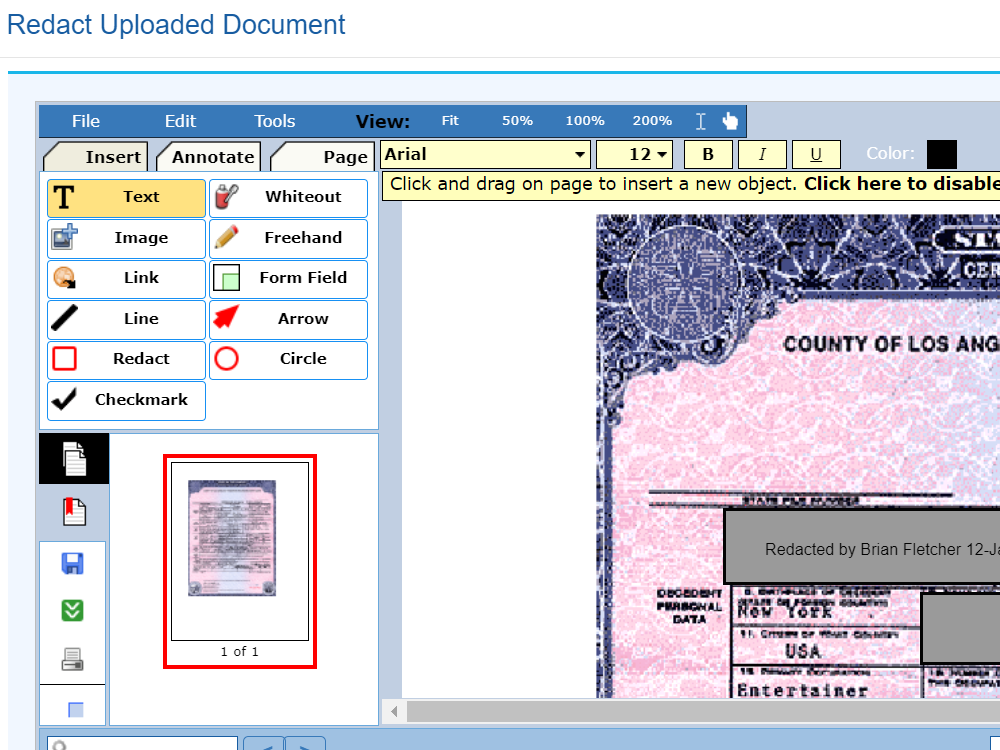
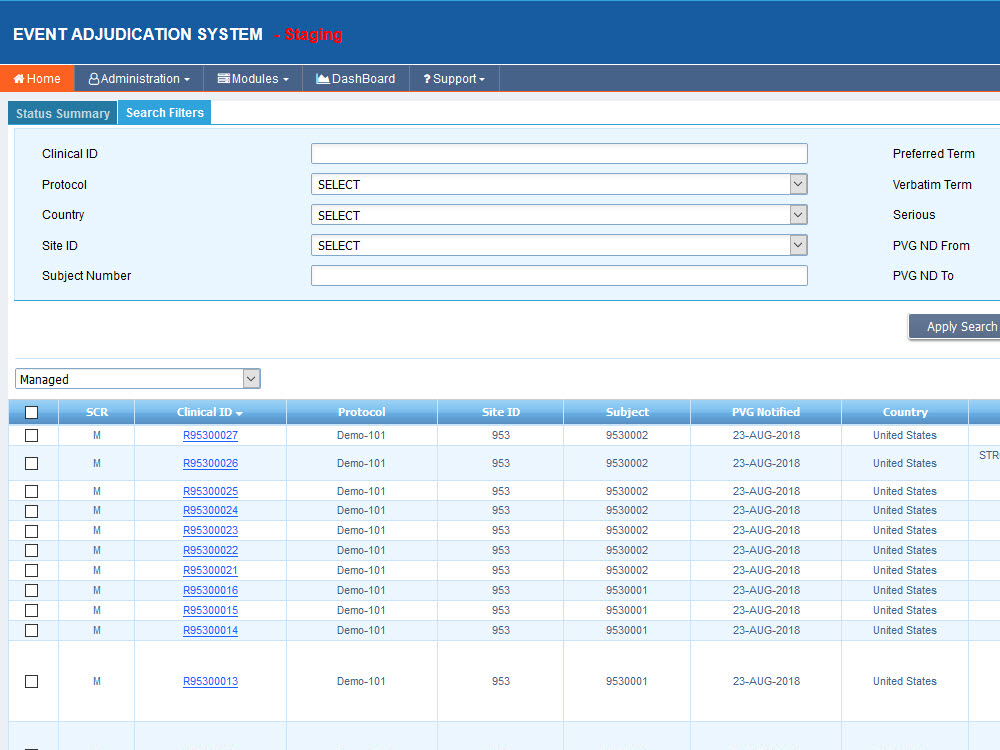
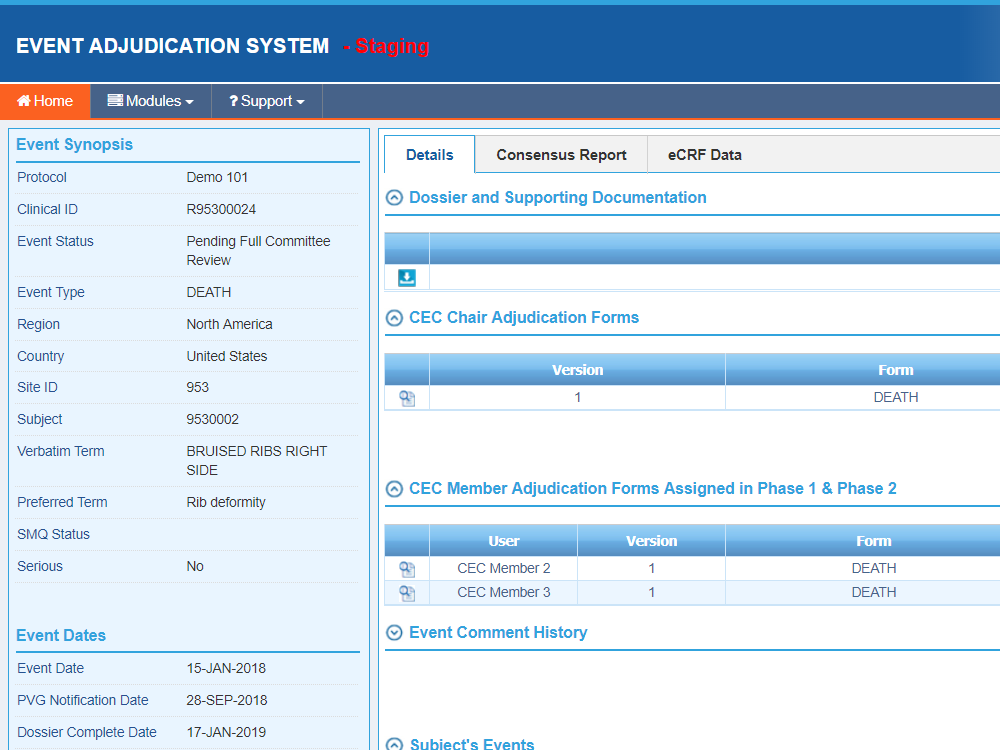
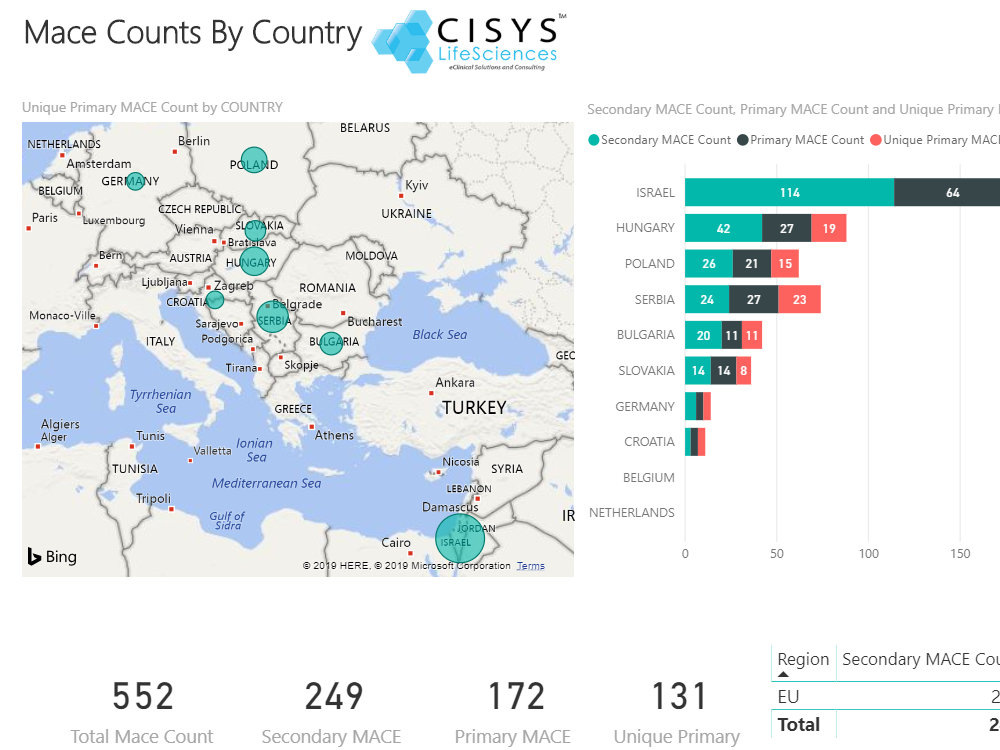
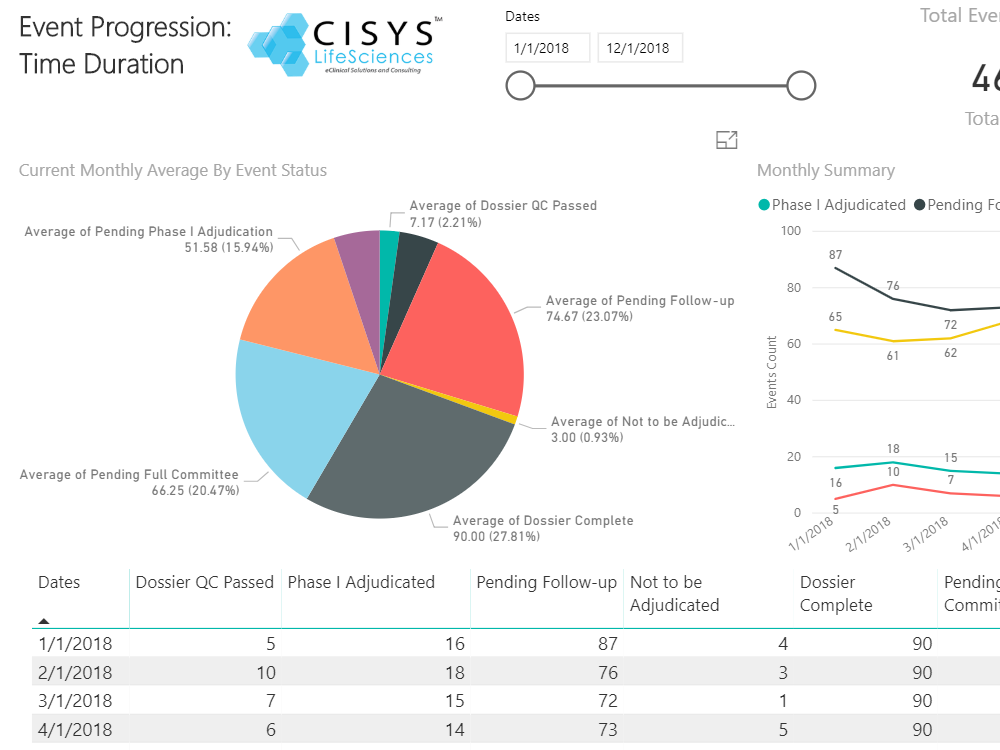
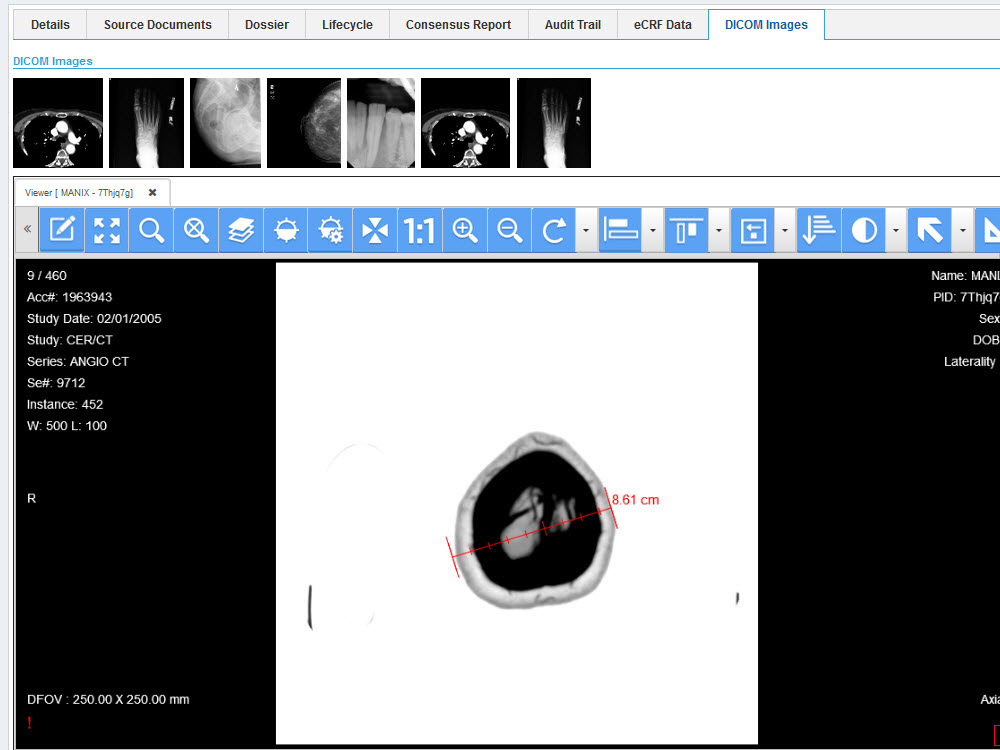
Sequence WebEAS is a highly configurable cloud based Clinical Event/Endpoint Adjudication platform that improves compliance and reduces costs for CROs, Sponsors and CEC organizations.
- AI Powered with Advanced Learning Algorithms
- Complex concordance and adjudication workflow
- Fully customizable workflows
- Intuitive PVG and committee member dashboards
- Robust search engine
- Integrated WebFXP Site Portal for Document Exchange and communication between Sites and Safety groups
- Powerful edit checks/data validation for Classification Forms
- Automated dossier compilation
- Committee Meeting Notes Management Module
- SMQ’s for consistent event identification
- Committee Member payment tracking
- Extensive reporting and metrics
- Improves compliance & ensures adherence to procedures for unbiased review of clinical data
- Lite Version: Price Points designed to replace paper studies
- Provides cost and timeline reduction to all parties involved in the clinical adjudication process
- Eliminates adjudication backlog by efficiently capturing accurate and pertinent information needed to classify events
- Minimal training required
- Manage Meetings, Notes, attendees and all ancillary data for review history
- Eliminates the rigidity of other adjudication platforms
- Streamlines source document collection and automates dossier creation and bookmarking
- Fully customizable to eliminate the need for out-of-system workarounds and loss of efficiency
- Greatly increases efficiency for PVG, Committee, and Bio-statistician personnel
- CDISC / CDASH standards
- 21 CRF Part 11 Compliant
WebEAS Advanced
- Complex/customized concordance and adjudication workflow
- All features: SMQ, WebFXP, Dicom, EDC integration, MACE, Meeting Management module, etc.
- Fully customizable to eliminate the need for out-of-system workarounds and loss of efficiency
WebEAS Standard
- Configurable adjudication and concordance workflow
- Features: SMQ, WebFXP, EDC integration, MACE
WebEAS Lite
- Simple adjudication workflow and features
- Limited event counts
- Price Point designed to replace paper studies
Support and Training
- Quick and efficient user training
- In-person training also available onsite at sponsor facilities
- Zero client requirements except browser
- Remote training available
- Technical support and dedicated 800 number per study
- Many levels of help desk and support options available
Security
- Role and workflow-based security
- Customizable event notifications
- Configurable workflow to fit customer’s organization
- Configurable down to the field level
- Configurable actions by role or user
Data / Hosting
- All data will be hosted on a validated, secure server located in a hardened, redundant hosting facility
- Full business continuity and disaster recovery functions provided
- All data will be backed up on a nightly basis both locally and to a different geographic location for redundancy
- 24/7/365 Performance and fault monitoring
- All files will be the client’s property and transferred upon request